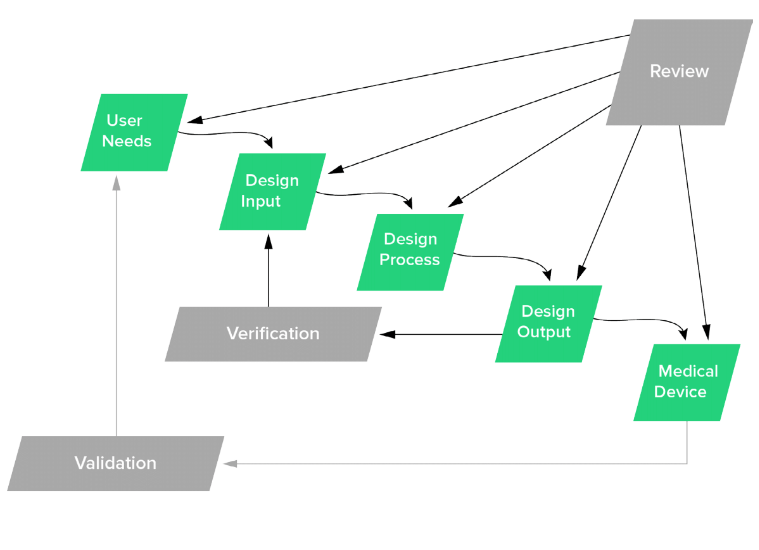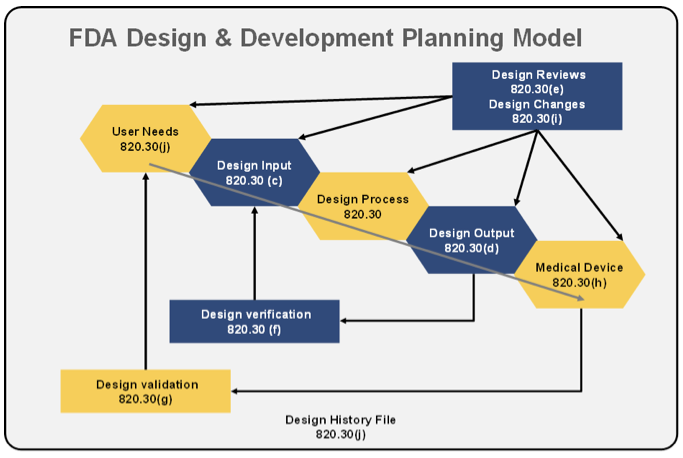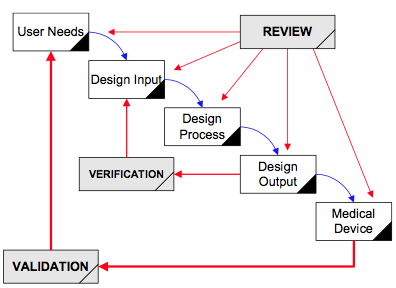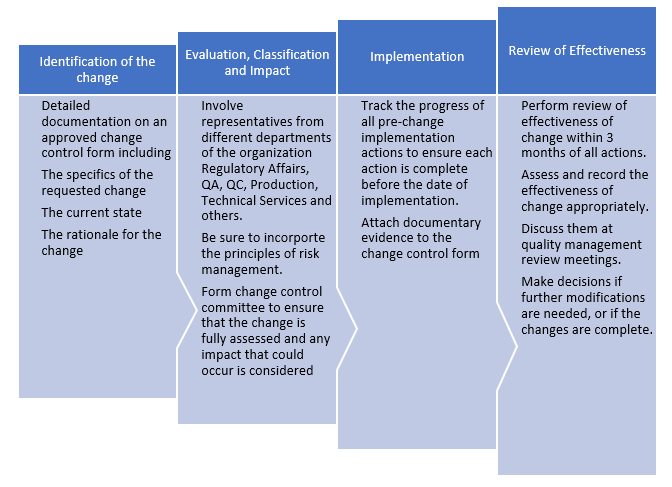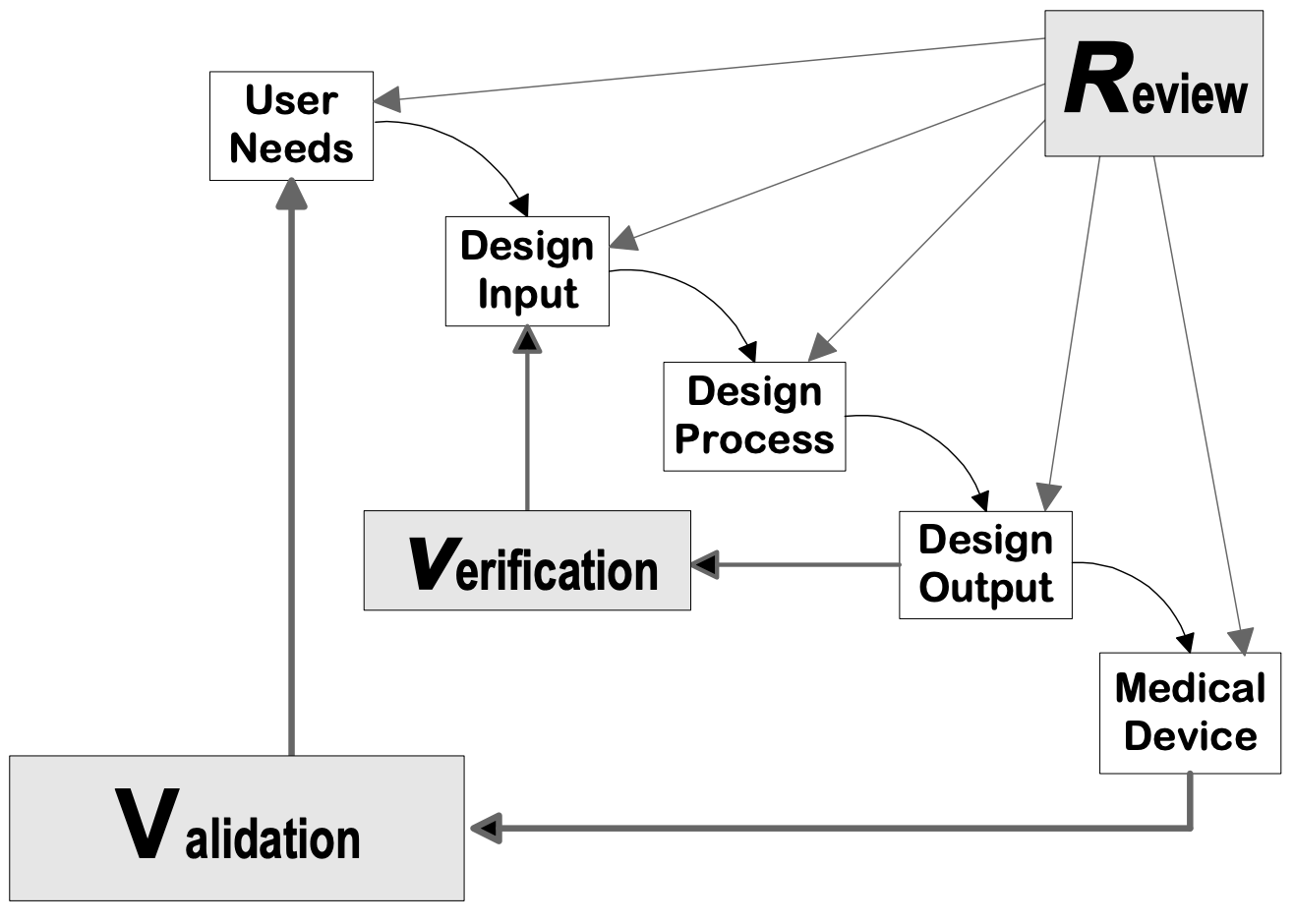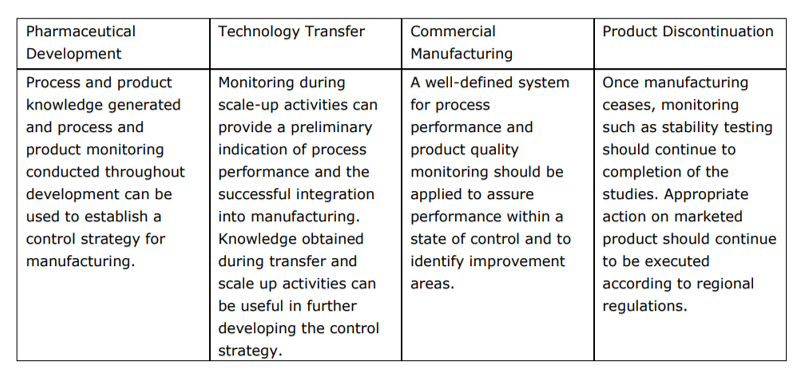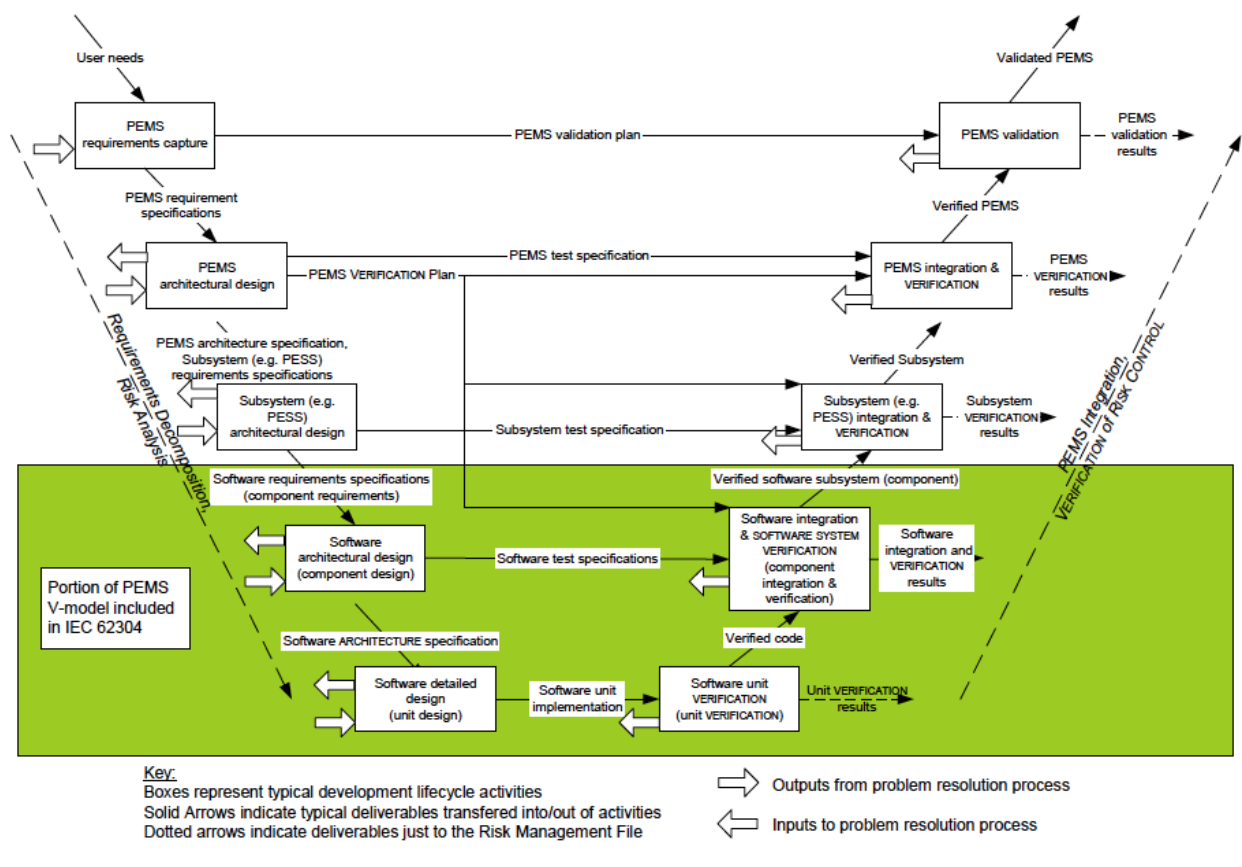
Q8, Q9, & Q10 Questions and Answers -- Appendix: Q&As from Training Sessions (Q8, Q9, & Q10 Points to Consider) | FDA

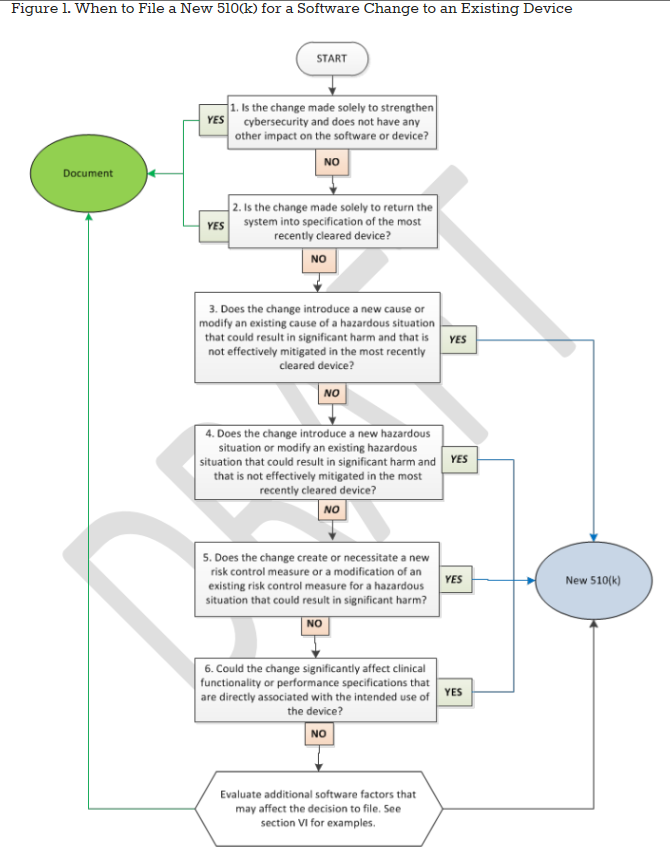
ICH Q12 Examples – Pharmaceutical Product Lifecycle Management Examples – FDA Guidance – Quality by Design for Biotech, Pharmaceutical and Medical Devices